Stryker’s Trident® Ceramic Hip Replacement System
In 1999, Stryker advanced the design of hip implants with the ceramic-on-ceramic and titanium hip, representing the latest innovations in this technology with the Trident® hip replacement system. Unlike conventional hip replacement systems, the Trident® System utilizes alumina ceramic-on-ceramic surfaces rather than metal-on-plastic or metal-on-metal. Ceramic-on-ceramic components have demonstrated significantly lower wear versus the conventional systems in the laboratory. Therefore, it is anticipated that these improved wear characteristics will potentially extend the life of the implant.
The Trident® Ceramic System is a cementless system, which means that the materials are fixed to the bone through implant design and technique, rather than using bone cement. Additional technology such as arc deposition and hydroxyapatite (HA) coating are an essential part of the shell design.
- Arc deposition is a titanium metal-coating process which is applied to the outer surface of the Trident® acetabular shell. It provides a roughened, textured surface on the acetabular shell, which helps provide initial fixation of the implant onto the bone.
- Hydroxyapatite (HA) is a naturally occurring substance that closely resembles natural bone mineral. Bone mineral stores the body’s supply of calcium and phosphorous — two minerals critical to one’s health. In fact, the two major components of HA are calcium and phosphorus — the predominant components of bone and tooth enamel. It’s no wonder that the material is so biocompatible and has enjoyed great success in orthopaedic and dental applications for over two decades.
Advantages of Stryker’s Trident® Ceramic System:
- Potential to wear less, last longer. The insert and head of the Trident® System are both alumina ceramic, which has demonstrated significantly lower wear versus conventional plastic-on-metal joint systems in the laboratory. It is anticipated that the improved wear characteristics of alumina ceramic may result in a longer lasting implant.1
- It’s stronger than other ceramic hip systems on the market. The Trident® Ceramic System has a unique design that features a patented titanium sleeve surrounding the ceramic acetabular shell. The titanium sleeve increases the material strength of the ceramic insert by 50% versus other available ceramic inserts.2
- Biocompatible. The type of ceramic used in total hip replacement today is aluminum oxide, also known as alumina. Alumina ceramic is extremely compatible with the body.
Range of motion for the Trident® Ceramic System
Many people are concerned that they’ll experience a more limited range of motion following total hip replacement. Most want to regain sufficient motion so that they can return to everyday activities, such as climbing stairs and sitting or bending to tie their shoelaces, without concern for dislocation. Although there are many factors that will influence your range of motion, the Trident® Ceramic System is designed to accommodate the range of motion of a normal, healthy hip joint. An increased range of motion may minimize the risk of hip dislocation.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker and Trident. All other trademarks are trademarks of their respective owners or holders.
References:
1. Taylor SK, Serekian P, Manley M. “Wear Performance of a Contemporary Alumina: Alumina Bearing Couple Under Hip Joint Simulation.” Trans. 44th Ann. Mtg. ORS, 51, 1998.
2. Stryker Test Report MT0014.
System Description
The Trident® Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The artificial hip replacement device consists of four basic components: an alumina ceramic insert (socket liner), an alumina ceramic femoral head (ball), a metal acetabular shell (socket), and a metal femoral stem (hip stem).
The hip stem is inserted into the top of the thighbone. The ball fits onto the top of the hip stem. The socket liner and mating socket are fixed to the hip joint. The ball and socket articulate together.
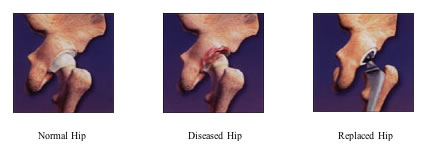
The Trident® implant has bearing surfaces (the ball and socket) made of alumina ceramic. Laboratory testing of alumina ceramic has shown it to have less wear than the metal and plastic materials that are currently used in total hip surgery. Alumina ceramic is extremely hard — in fact, its hardness is second only to diamond — and provides excellent lubrication between the ball and socket. Because of its material characteristics, alumina ceramic-on-ceramic demonstrates significantly lower wear versus conventional metal-on-plastic components in laboratory testing. Therefore, it is anticipated that these improved wear characteristics will extend the life of the implants.
Purpose of the Device
This total hip replacement is indicated for patients with painful, disabling hip joint disease caused by a severe form of arthritis.
This total hip replacement should not be used for patients:
- Who may have an infection in or near the hip joint,
- Who are unable to comply with the instructions for preparing for and recovering from the surgery,
- Who do not have enough healthy bone to support fixation of the implants, or
- Whose bones are not fully grown.
Clinical Experience
Howmedica Osteonics Corp. (hereafter referred to as Stryker Orthopaedics) conducted a clinical study in the United States for its ceramic-on-ceramic hip system, which was conducted under a study protocol approved by the United States Food and Drug Administration. The study was performed at 16 orthopaedic centers of excellence across the United States. The clinical study was conducted for Stryker Orthopaedic’s first generation ceramic-on-ceramic design (called the ABC System), and second generation ceramic-on-ceramic design (called the Trident® System). In all, the data from 558 hip replacement surgeries (cases) with these ceramic-on-ceramic bearings were evaluated.
The prospective, randomized, clinical study began in 1996, with 349 cases with the first-generation ceramic-on-ceramic systems, and 165 cases with a control device (conventional, metal-on-polyethylene). The clinical data on these cases were evaluated out to 24 months (2 years) after surgery. In 1999, the patented Trident® Ceramic System was then entered into the study. This patented design offers a stronger, easier-to-use ceramic liner design. Two hundred nine (209) Trident® inserts were implanted in the study. The clinical data from these cases has also been evaluated out to 24 months (2 years) after surgery.
The Trident® System features the identical ceramic-on-ceramic articulating bearing surface as the ABC System; however, the Trident® system features an outer titanium sleeve. The titanium sleeve offers the following unique advantages over other first generation ceramic-on-ceramic designs:
- Increases the strength of the ceramic insert by 50%: The Trident® Alumina Insert is the strongest ceramic insert on the U.S. market today,
- Protects the insert rim from chipping during implant assembly: Occasional chipping of the insert rim during surgery was reported during clinical studies of first-generation designs. No Trident® ceramic implants chipped or fractured in the study.
- Allows reassembly of the acetabular shell to the insert: First-generation designs prohibit reassembly of a ceramic insert to its shell, making insert adjustment or replacement more difficult.
Safety Data
Safety of the ceramic-on-ceramic hip systems was established by studying the adverse (unfavorable) events within the clinical study. The adverse events experienced by patients who received the ceramic-on-ceramic hip systems (test group) were comparable to the adverse events experienced by patients who received a conventional, metal-on-polyethylene hip system (control group). There were no deaths in the test or control study groups. Additional surgery to replace or remove components occurred 4 times in the Trident® ceramic-on-ceramic group, as compared to 5 times in the metal-on-poly (control group). Analysis of the adverse event data demonstrated that there was no significant difference between the adverse events experienced in the test and control groups.
Effectiveness Data
The effectiveness of the Trident® ceramic-on-ceramic systems was established by comparing the Harris Hip Scores (HHS) and the radiographic (X-ray) measurements of patients who received the ceramic-on-ceramic systems to those of patients who received the metal-on-polyethylene (control) hip system.
Pain and Function Improvement:
The Harris Hip Score is a scale from 1-100 that assesses a patient’s level of pain and function. The highest possible score (100) indicates pain relief and normal functional ability. The lowest possible score (0) indicates severe pain and disability. A score of 90-100 is considered excellent. At two years after surgery, the average Harris Hip Scores for the ceramic-on-ceramic group and for the control group were both in the excellent range.
X-ray (Radiographic) Measurements:
X-rays were reviewed at regular intervals after surgery to look for signs of possible device loosening, device movement, or accelerated wearing away of the components. At two years after surgery, all of the 185 Trident® cases evaluated were considered a radiographic success. No devices showed signs of device loosening, device movement, or accelerated wearing away of the components.
Patient Success Rates:
A patient was considered a success within the study if, at two years after surgery, the hip implant system was still in place (had not been replaced), the Harris Hip Score was greater than 70 points, and there were no x-ray signs that might indicate loose or unstable hip implant components. The patient success rate for the control group was 94%. The patient success rate for the Trident® ceramic-on-ceramic group was 97%.
Use for Inflammatory Joint Disease
The study results presented above include only patients who had primary total hip replacement for severe, non-inflammatory degenerative joint disease. Eight additional cases of inflammatory joint disease were enrolled in the study, and received a ceramic-on-ceramic system (either ABC or Trident®). The eight cases have been followed for a mean duration of 16 months. As of the latest functional evaluations, the mean HHS is 94 (range 85-100). There have been no reoperations or revisions. There have been no operative-hip-related complications. All components appear stable on X-ray.
Potential Benefits
The goals of artificial hip replacement include relief of pain, restoration of function, and correction of deformity. Ceramic-on-ceramic, however, demonstrates significantly lower wear versus conventional metal-on-plastic components in laboratory testing. Therefore, it is anticipated that these improved wear characteristics will result in a longer lasting implant.
Potential Risks
Any artificial hip replacement may be associated with serious complications. These include: dislocation, loosening, implant breakage, bone fracture, reaction to the implant’s materials, bone loss, change in the length of the treated leg, pain, hip stiffness, excessive bleeding, hip joint fusion, nerve damage, allergic reaction to medical and/or blood transfusion, infection, reactions to pain-relieving drugs, blood clots in the legs and/or lungs, amputation, heart attack, pneumonia, excessive wear of the implant’s components, decrease of bone mass, and audible sounds during motion.
With this ceramic-on-ceramic system, sudden breakage of ceramic components resulting from excessive forces is possible; however, no ceramic component broke during the clinical study. Corrosion (eroding) between the insert and shell may be possible; however, this event was not demonstrated in the clinical study, and laboratory tests have shown the potential to be minimal.
An audible noise during motion, such as a squeak, has been reported for patients receiving a ceramic-on-ceramic bearing couple. A 0.5% rate of squeaking noise has been reported in the clinical study with the Trident® Alumina Insert.
Any of the above-cited complications may require medical intervention, including additional surgery. In rare instances, complications may lead to death. Please ask your surgeon to discuss with you any of these complications that are not familiar to you.
Patient Instruction
Call your doctor if you experience any of the following symptoms:
- Redness, burning, swelling, or drainage from your operated area
- Fever of 100 degrees or higher
- Pain that does not lessen with rest
- Acute, severe pain in the hip associated with twisting, turning or injury
Consult your doctor regarding considerations before surgery, rehabilitation after surgery, and expectations for surgery. It is important to begin planning for your return home from the hospital before your surgical procedure. Your surgeon may suggest tips to prepare your home for after surgery. For example, get an apron or belt with pockets to carry things while you are on crutches, buy or borrow a cordless phone, remove scatter rugs and other obstacles to safe transport using crutches, have high chair and commode accessories available. Above all, during this time, treat yourself well, eat balanced meals, get plenty of rest, and if requested by your surgeon, donate your own blood so it can be transfused during and after surgery.
After surgery you will need to rest your hip to allow proper healing. Your activity will be restricted during this healing period. During the first weeks after surgery, you may be advised to put a pillow between your legs when turning over in bed, wear elastic stockings, use raised toilet seat, take showers rather than baths, restrict activities such as sudden twisting or turning, crossing legs, exposing the scar to sunlight, and driving. Follow carefully your doctor’s instructions regarding progression to normal weight bearing and resumption of normal physical activity. Individual results will vary and all patients will experience different activity levels post-surgery.
Even after the healing period, excessive loads placed on the implants through sudden trauma or high impact activities, such as running and jumping, can damage the artificial joint. While the expected life of an artificial hip replacement system is difficult to estimate, it is finite. The components are made of foreign materials that will not indefinitely withstand the activity level and loads of normal, healthy bone. The hip joint may have to be replaced at some time in the future.
Alternative Treatments
Other options may include use of a conventional hip replacement system, other surgical procedures that do not replace the hip joint, or non-surgical treatments based on pain management and activity restriction. Your doctor can explain these alternatives, and help you decide which treatment is best for your condition.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Stryker and Trident. All other trademarks are trademarks of their respective owners or holders.
References:
1. Taylor SK, Serekian P, Manley M. “Wear Performance of a Contemporary Alumina: Alumina Bearing Couple Under Hip Joint Simulation.” Trans. 44th Ann. Mtg. ORS, 51, 1998.
2. Stryker Test Report MT0014.